EN - Self study - PECB Certified ISO 13485 Foundation
Become acquainted with the best practices of Medical Devices Quality Management Systems (MDQMS) based on ISO 13485
Exam voucher: Included (valid for 12 months) + 2nd try |
Course material e-book: Included |
Video training: Included for eLearning courses only |
What is included?
- You have 12 months time as of reception of the learning material to learn, do the exam and get your certification
- Certification and examination fees are included in the price of the training course
- Participants will be provided with the training course material containing over 200 pages of explanatory information, examples, best practices, exercises, and quizzes.
- An Attestation of Course Completion worth 14 CPD (Continuing Professional Development) credits will be issued to the participants who have attended the training course.
- In case candidates do not pass the exam, they are entitled to a free retake within 12 months from the date the coupon code is received
- + 20% reduction on the first year subscription for our all-in-one ISMS/GRC management solution
Why should you attend?
ISO 13485 Foundation training enables you to learn the basic elements to implement and manage a Medical Devices Quality Management System (MDQMS) as specified in ISO 13485. During this training course, you will be able to understand the different modules of a MDQMS, including MDQMS policy, procedures, performance measurements, management commitment, internal audit, management review and continual improvement.
After completing this course, you can sit for the exam and apply for the “PECB Certificate Holder in ISO 13485 Foundation” certificate. A PECB Foundation Certificate shows that you have understood the fundamental methodologies, requirements, framework and management approach.
Who should attend?
- Individuals involved in Medical Devices Quality Management
- Individuals seeking to gain knowledge about the main processes of Medical Devices Quality Management Systems (MDQMS)
- Individuals interested to pursue a career in Medical Devices Quality Management
Learning objectives
- Understand the elements and operations of a Medical Devices Quality Management System (MDQMS)
- Acknowledge the correlation between ISO 13485 and other standards and regulatory frameworks
- Understand the approaches, methods and techniques used for the implementation and management of a MDQMS
Educational approach
- Lecture sessions are illustrated with practical questions and examples
- Practical exercises include examples and discussions
- Practice tests are similar to the Certificate Exam
Course agenda
- Module 1: Introduction to Medical Devices Quality Management System (MDQMS) concepts as required by ISO 13485
- Module 2: Medical Devices Quality Management System requirements and Certification Exam
Examination
The exam fully meets the requirements of the PECB Examination and Certificate Programme. It covers the following competency domains:
- Domain 1: Fundamental principles and concepts of a Medical Devices Quality Management System (MDQMS)
- Domain 2: Medical Devices Quality Management System (MDQMS)
For specific information about exam type, languages available, and other details, please visit the List of PECB Exams and the Examination Rules and Policies.
Certificate requirements
After successfully completing the exam, you can apply for the credential shown on the table below.
For more information, please refer to the Certification Rules and Policies.
The certificate requirements for the ISO 13485 Foundation are:
| Designation | Exam | Professional experience | MS audit/assessment experience | MDQMMS project experience | Other requirements |
| PECB Certificate Holder in ISO 13485 Foundation | Pass the PECB ISO 13485 Foundation Exam | None | None | None | Signing the PECB Code of Ethics |
Contact us on [email protected] if you have other questions
Start for free now!
Streamline your GRC work using our all-in-one management solution and get access to our network of local specialists
Ask any question about our products
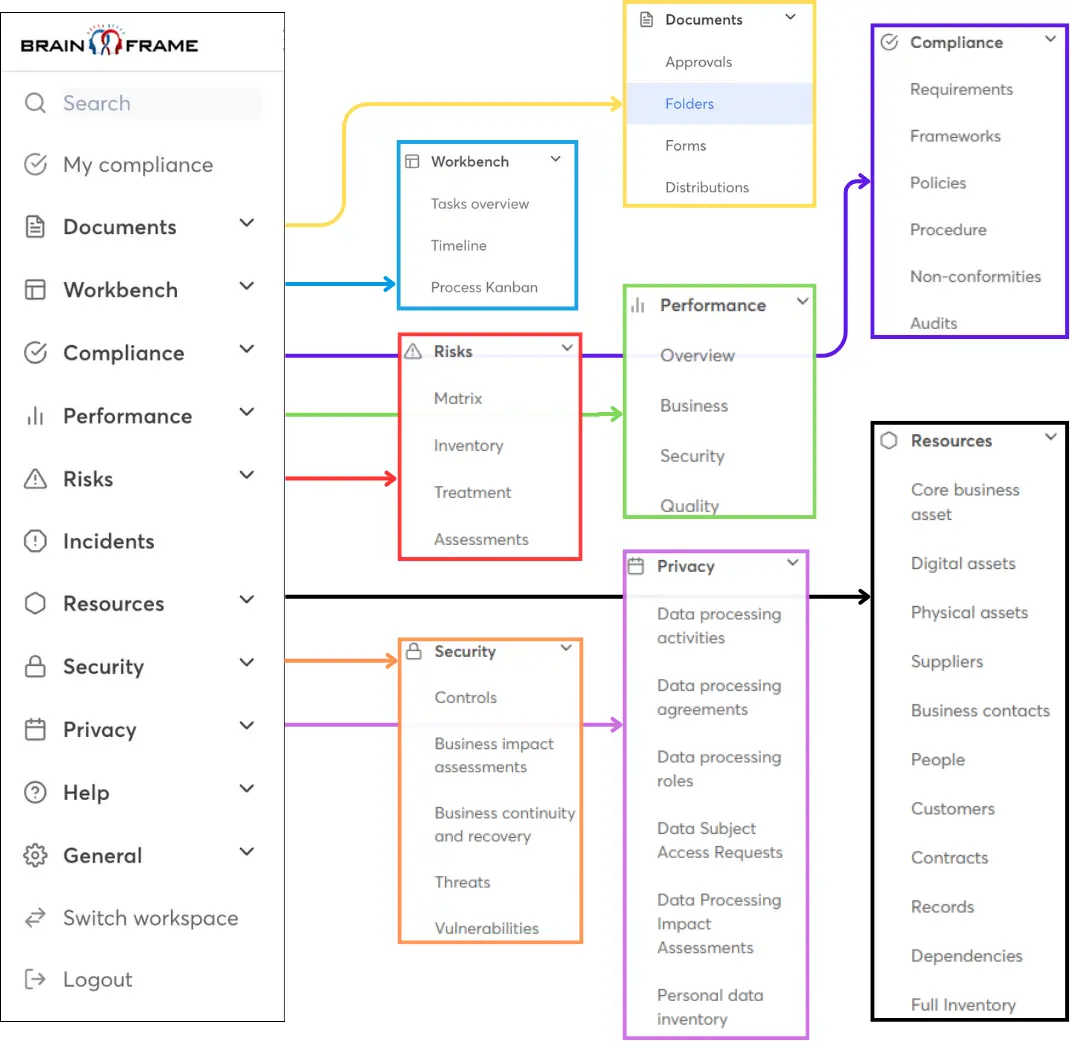
Streamline your GRC work using our all-in-one management solution and get access to our network of local specialists
Start your free account
