EN - Self study - PECB Certified ISO/IEC 17025 Lead Implementer
Master the implementation and management of Laboratory Management Systems (LMS) based on ISO/IEC 17025
Examenbon: Inbegrepen (geldig voor 12 maanden) + 2e poging |
Cursusmateriaal e-boek: Inbegrepen |
Video materiaal: Enkel inbegrepen voor eLearning cursussen |
What is included?
- You have 12 months time as of reception of the learning material to learn, do the exam and get your certification
- Certification and examination fees are included in the price of the training course
-
Training material containing over 400 pages of information and practical examples will be distributed
- An Attestation of Course Completion worth 31 CPD (Continuing Professional Development) credits will be issued to the participants who have attended the training course.
- In case candidates do not pass the exam, they are entitled to a free retake within 12 months from the date the coupon code is received
- + 20% reduction on the first year subscription for our all-in-one ISMS/GRC management solution
Why should you take this training course?
ISO/IEC 17025 Lead Implementer training enables you to develop the necessary expertise to support laboratories to meet the requirements of the standard by implementing a quality control system based on ISO/IEC 17025. During this training course, you will also gain a thorough understanding of the best practices of an LMS in testing and calibration laboratories in order to produce reliable measurement results and open your laboratory`s doors for accreditation and international recognition.
After mastering all the necessary concepts of Laboratory Management Systems, you can sit for the exam and apply for a “PECB Certified ISO/IEC 17025 Lead Implementer” credential. By holding a PECB Lead Implementer Certificate, you will be able to demonstrate that you have the practical knowledge and professional capabilities to implement ISO/IEC 17025 in your laboratory.
Who should attend?
The ISO 13485 Lead Implementer training course is intended for:
- Individuals involved in Testing and Calibration Laboratories
- Managers or consultants seeking to master the implementation of a Laboratory Management System
- Laboratory technicians responsible for maintaining conformance with Testing and Calibration Laboratories Accreditation requirements
- Individuals responsible for supporting the operations of a Testing and Calibration Laboratory
- Technical experts seeking to prepare for Testing and Calibration Laboratories competence assessment
Training course structure
Module 1: Introduction to ISO/IEC 17025 and initiation of a LMS
- Course objectives and structure
- Standards and regulatory frameworks
- Laboratory Management System
- Basic Concepts in Testing and Calibration
- Initiating the MDQMS implementation
- Understanding the organization and clarifying the quality objectives
- Analysis of the existing system
Module 2: Plan the implementation of the MDQMS
- Leadership and approval of the MDQMS project
- LMS scope
- Laboratory policies
- Definition of the organizational structure
- Definition of the document management process
Module 3: Implementation of a MDQMS
- Design of controls and drafting of specific policies & procedures
- Communication plan
- Training and awareness plan
- Resource Management
- Customer Management
- Operations Management
Module 4: LMS monitoring, measurement, continuous improvement and preparation for accreditation
- Monitoring, Analysis, and Evaluation
- Internal audit
- Management review
- Treatment of problems and non-conformities
- Continual improvement
- Preparing for the accreditation
- Competence and evaluation of implementers
- Closing the training
Certification Exam
Learning objectives
Upon successfully completing the training course, you will be able to:
- Acknowledge the correlation between ISO 13485 and other standards and regulatory frameworks
- Master the concepts, approaches, methods and techniques used for the implementation and effective management of a Laboratory Management System (LMS)
- Learn how to interpret the ISO/IEC 17025 requirements in the specific context of the laboratory
- Learn how to support a laboratory to perform testing and other operating activities
- Acquire the expertise to advise a laboratory in implementing Laboratory Management System best practices
Examination
The “PECB Certified ISO 28000 Lead Auditor” exam fully meets the requirements of the PECB Examination and Certification Program (ECP). It covers the following competency domains:
- Domain 1: Fundamental principles and concepts of a Laboratory Management System (LMS)
- Domain 2: Laboratory Management System (LMS)
- Domain 3: Planning a LMS based on ISO/IEC 17025
- Domain 4: Implementing a management system based on ISO/IEC 17025
- Domain 5: Performance evaluation, monitoring and measurement of a MDQMS based on ISO 13485
- Domain 6: Continual improvement of a LMS based on ISO/IEC 17025
- Domain 7: Preparing for an accreditation
Duration: 3 hours
Location: Online through the PECB app OR in person in one of the PECB exam centers
Preparation: PECB Exam Preparation Guides
Language: The exam is available in multiple other languages and does not need to be taken in the same language as the training material. Additional time can be requested when your native language is not available in your mother tongue (to be requested by candidates on the exam day)
Retake: In case you fail the exam, you can retake it within 12 months following the initial attempt for free
For specific information about the exam type, languages available, and other details, please visit the List of PECB Exams and the Examination Rules and Policies.
Certification?
After successfully completing the exam, you can apply for the credentials shown on the table below. You will receive a certificate once you comply with all the requirements related to the selected credential.
For more information about the ISO 28000 certifications and the PECB certification process, please refer to the Certification Rules and Policies..
The requirements for PECB Implementer Certifications are:
| Credential | Exam | Professional experience | MDQMMS project experience | Other requirements |
| PECB Certified ISO 13485 Provisional Implementer | PECB Certified ISO 13483 Lead Implementer Exam or equivalent | None | None | Signing the PECB Code of Ethics |
| PECB Certified ISO/IEC 17025 Implementer | PECB Certified ISO 13483 Lead Implementer Exam or equivalent | Two years: One year of work experience in to Laboratory Management | Project activities: a total of 200 hours | Signing the PECB Code of Ethics |
| PECB Certified ISO/IEC 17025 Lead Implementer | PECB Certified ISO 13483 Lead Implementer Exam or equivalent | Five years: Two years of work experience in Laboratory Management | Project activities: a total of 300 hours | Signing the PECB Code of Ethics |
| PECB Certified ISO 13485 Senior Lead Implementer | PECB Certified ISO 13483 Lead Implementer Exam or equivalent | Ten years: Seven years of work experience in Laboratory Management | Project activities: a total of 1,000 hours | Signing the PECB Code of Ethics |
To be considered valid, these implementation activities should follow best implementation practices and include the following activities:
- Drafting a MDQMS plan
- Initiating a MDQMS implementation
- Implementing a LMS
- Monitoring and managing a MDQMS implementation
- Performing continual improvement measures
Contact us on [email protected] if you have other questions
Start for free now!
Streamline your GRC work using our all-in-one management solution and get access to our network of local specialists

Start for free now!
Streamline your GRC work using our all-in-one management solution and get access to our network of local specialists
Stel eender welke vraag over onze producten
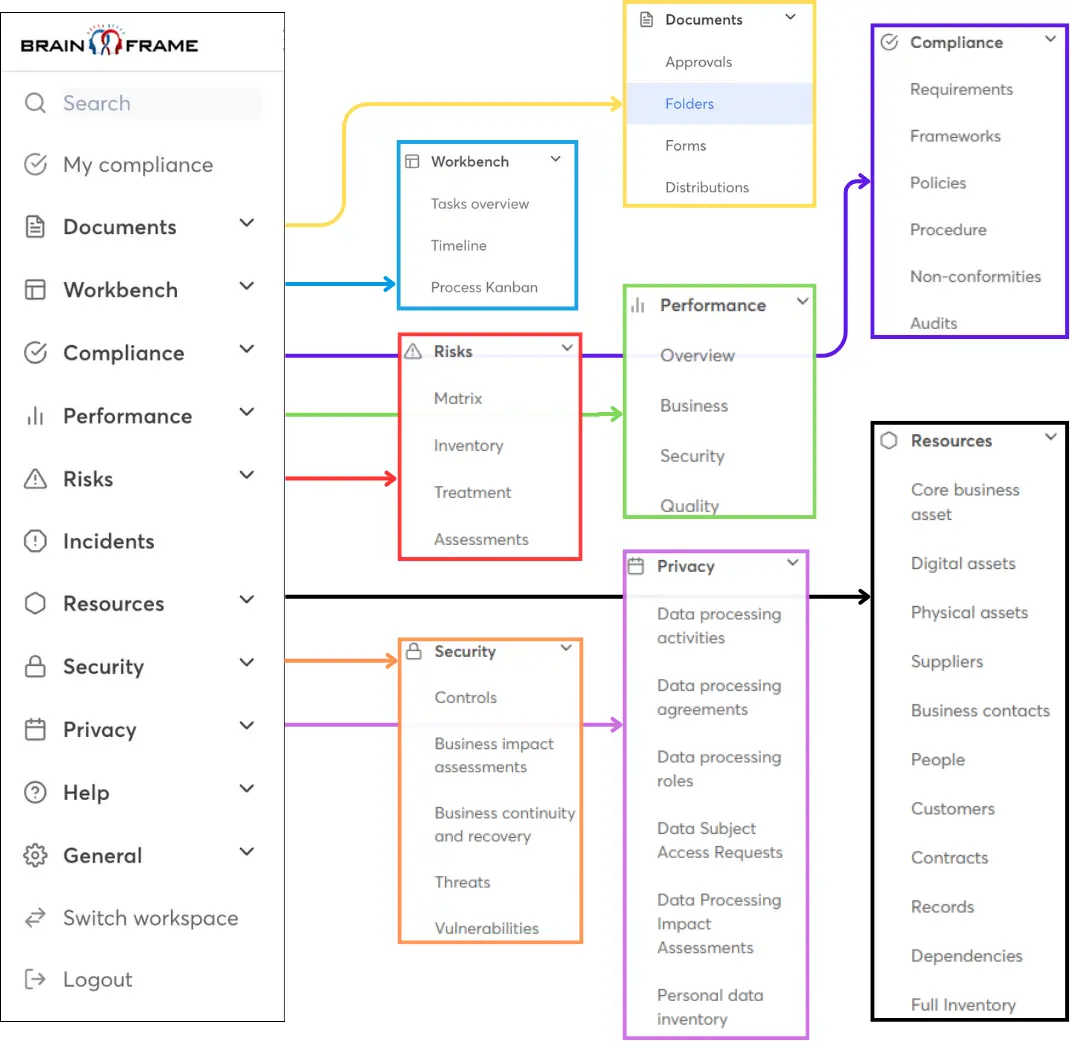
Stroomlijn uw GRC-werk met onze alles-in-één managementoplossing en krijg toegang tot ons netwerk van lokale specialisten
Start je gratis account
